On November 25, 2024, BrioHealth's U.S. subsidiary, BrioHealth Solutions, Inc., a wholly-owned BrioHealth's U.S. subsidiary, announced its independently developed fully magnetic levitation left ventricular assist device, BrioVAD®, had successful completed the first patient successful enrollment of the first patient in the INNOVATE clinical trial of its fully magnetically levitated left ventricular assist device, BrioVAD®, at Emory University Hospital.

Dr. Chen Chen, founder of BrioHealth and CEO of BrioHealth Solutions, shared his excitement: “We're thrilled to kick off the INNOVATE clinical trial. After years of hard work, innovation, and fine-tuning, BrioVAD® is finally here. Even though there have been advances in ventricular assist devices,, there's still a pressing need to improve device performance and patient outcomes. BrioHealth is committed to making that happen. We're really encouraged by the enthusiasm from the Pparticipationng Ccenters to push forward treatments for advanced heart failure."
Founded in 2008, BrioHealth has pioneered significantoriginal innovations over the past decade. The company successfully developed the BrioVAD®Cherish-VAD (mmodel: CH-VAD), an new generation ultra-compact, fully magnetically levitationed artificial heart, which became the first device of its kind to receive approval from China’s National Medical Products Administration (NMPA). To date, more than 350 patients in China have benefited from CH-VAD, showcasing outstanding clinical results. BrioVAD® utilizes the same implantable blood pump as CH-VAD, andbut includes many technological advancements breakthroughs that enhance the portability of external components portability and overall system performance.
The INNOVATE trial is a prospective, open-label, randomized, controlled, multicenter pivotal study aimed at assessing evaluating the safety and efficacy of the BrioVAD® system in treating advanced, refractory left ventricular heart failure.

Otto Gago, Chair Professor of Cardiac Surgery at the University of Michigan Medical School, and Dr. Francis D. Pagani, Dr. Francis D. Pagani, Otto Gago Endowed Professor of Cardiac Surgery at the University of Michigan Medical School and the lead researcherinvestigator of this study, commented: “Patients with advanced heart failure have limited treatment options, with only one LVAD available in the U.S. Although there have been advances, LVAD patients still face various complications. The INNOVATE trial aims to determine whether the BrioVAD® system can help reduce these complications and improve the quality of life for patients with advanced heart failure.”

Dr. Mani Daneshmand, Andrew J. McKelvey Endowed Professor at Emory University School of Medicine and Director of the Emory Heart and Lung Transplantation and Mechanical Circulatory Support program, remarked: “The launch of the INNOVATE trial is a significant milestone in expanding treatment options for patients with advanced heart failure. We are excited to see how the BrioVAD® system will benefit these patients.”
The commencement of the BrioVAD® INNOVATE clinical trial in the U.S. marks a crucial advancement in the field of heart failure treatment worldwide and represents a significant step forward in BrioHealth's global strategy.
About the BrioVAD® System
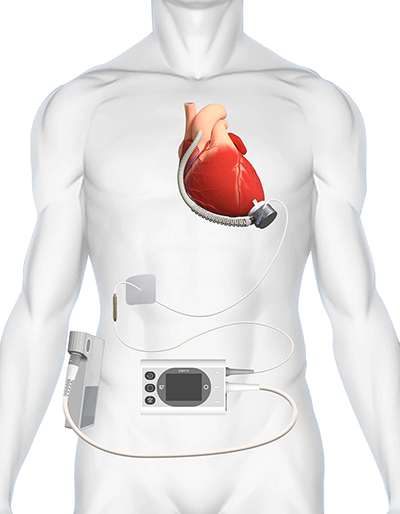
Illustration of the BrioVAD® Left Ventricular Assist System

Main Components of the BrioVAD® Left Ventricular Assist System
The BrioVAD® system is a durable left ventricular assist device (LVAD) designed to provide full-flow support for long-term use in heart failure patients. It consists of an implantable blood pump and external components. Unlike the only FDA-approved durable LVAD currently available in the U.S., the BrioVAD® features an innovative discrete magnetic levitation design that combines a compact pump body with a larger impeller, thereby minimizing surgical trauma. Additionally, the BrioVAD® employs a novel percutaneous cable that is thinner and more flexible, aimed at reducing the risk of infection.
The distinctive magnetic levitation structure and large-diameter impeller of the BrioVAD® pump provide a foundation for optimized internal flow paths. This advanced flow path design enhances blood compatibility and improves hemodynamic performance, ultimately lowering the risk of severe complications.
The external components of the BrioVAD® system are uniquely designed to improve user experience and patient quality of life. Patients only need to carry two components daily, significantly enhancing convenience and comfort.
About the INNOVATE Clinical Trial

The INNOVATE trial is a prospective, random controlled, open-label, multi-center pivotal study designed to assess the safety and effectiveness of the BrioVAD® system in patients with advanced, refractory heart failure needing either short-term or long-term mechanical circulatory support. In this study, participants will be randomly assigned in a 2:1 ratio to receive either the BrioVAD® system or the HeartMate 3. The primary endpoints are survival free from disabling stroke and pump replacement at both 6 months and 24 months. Secondary endpoints include evaluating adverse events, cardiac function, and quality of life in heart failure patients. The trial will also investigate whether implantation of the BrioVAD® can reduce the total number of hospital days during the follow-up period. Approximately 800 patients will be enrolled across leading medical centers in the United States. For more information, visit www.theinnovatetrial.com.

-
Baheal Medical Inc. Lands on ChiNext Board Today!
2021-06-30 -
2021-11-09
-
Baheal Medical New Retail Business: Cute Camel Store 2.0 Opened in Qingdao
2021-03-20 -
2021-11-26
-
BAHEAL Pharmaceutical Group Accepted by International Federation of Pharmaceutical Wholesalers
2016-04-05 -
Baheal Receives Four Honors at China Future Healthcare Rankings Top 100
2021-04-17 -
Baheal Medical Launches Commercialization Platform of Efficacious Cosmetics
2021-05-13 -
2019-03-29





