The National Healthcare Security Administration announced the revision of the National Reimbursement Drug List (NRDL) and the new version of the list on 18 January 2023. Osmotic Pump Metformin Hydrochloride Sustained-Release Tablets ®, a product of Qingdao Baheal Pharmaceutical Co., Ltd. (hereinafter referred to as "Baheal Pharma"), a subsidiary of Baheal Pharmaceutical Group, with its unique clinical value and dosage form advantages, has been successfully included in the National Reimbursement Drug List, which will greatly improve the accessibility and affordability of high-quality hypoglycemic drugs and benefit more diabetic patients.
There is currently a high incidence of diabetes worldwide. In China, there are approximately 140 million patients with diabetes, most of whom have type 2 diabetes mellitus (T2DM). Metformin is the first choice for the treatment of type 2 diabetes, and is also the first choice for monotherapy and combination therapy, with significant hypoglycemic effect. But unfortunately, the clinical use rate of metformin in China is only 53.7 per cent, a huge difference from the 70 to 80 per cent in developed countries. The main reason is that gastrointestinal side effects and administration errors occur due to the difference in preparation technology. Medication compliance is an important factor affecting clinical utilization rates.
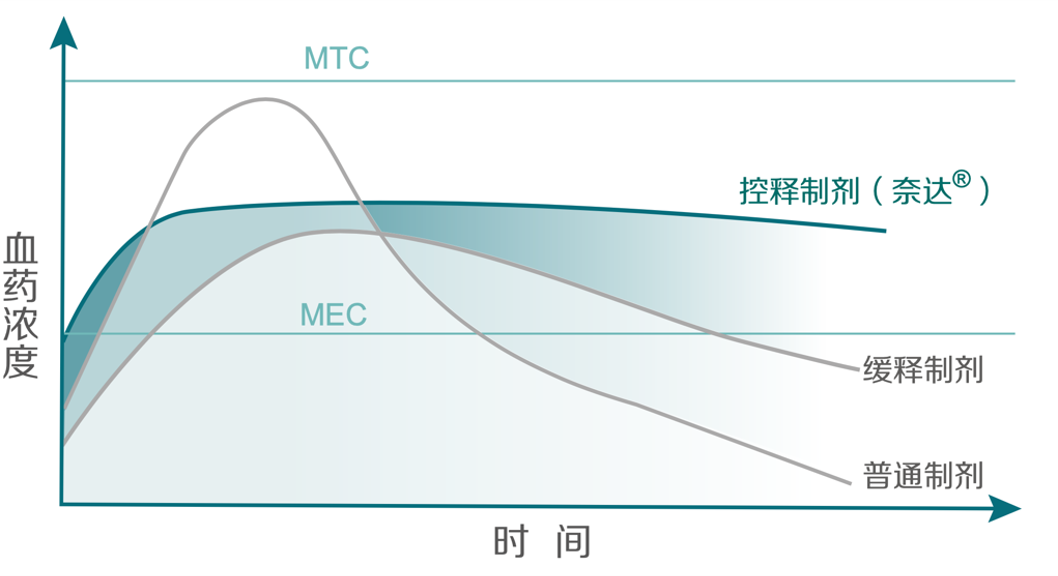
Baheal Pharma's Nida® incorporates the world's most advanced osmotic pump controlled release technology, which uses the osmotic pressure principle to release the drug's active ingredients at a constant rate to maintain blood concentrations within the effective range within 24 hours. While reducing gastrointestinal irritation and toxic side effects, it also reduces the number of medications taken by diabetics, requiring patients to take only once daily to maintain blood glucose stability, effectively improving patient compliance. At the same time, Nida matches the originator drug in active ingredients, dosage specifications, use and dosage, and bioequivalence.
It is worth noting that to break the patent blockade of osmotic pump controlled-release technology, Baheal Pharma spent 10 years focusing on research and development, and finally broke through this technical barrier. Nida ® has also passed the marketing approval of the US FDA and China's NMPA, which not only realized the domestic replacement of high-end preparations, but also "went to sea" for sale in the US market in 2019.
During the negotiation and communication with the health insurance companies, Baheal Pharma reduced the price of Nida to about 20% of the export price, so that people can have access to high-end preparations at advantageous prices. In addition, Baheal Pharma's memantine hydrochloride sustained-release capsules were approved in 2022 and entered the seventh batch of national centralized procurement, and this time are directly included in the national reimbursement drug list.
As the R&D and manufacturing platform of high-end controlled release preparations under Baheal Pharmaceutical Group, Baheal Pharma focuses on researching, developing, manufacturing and selling high-end controlled release preparations such as osmotic pump, pellet coating and matrix tablets. It has passed the US Food and Drug Administration's cGMP (Dynamic Drug Manufacturing Practice) inspection with "zero defects", and many products have achieved equal registration and marketing in China and the US. Previously, Baheal Pharma has developed and produced products such as celecoxib capsule Neqi, duloxetine hydrochloride enteric capsule Neshu, nifedipine controlled release tablets and memantine hydrochloride sustained release capsule Nexin.
In recent years, the National Healthcare Security Administration has continuously promoted the adjustment of the National Reimbursement Drug List, supported and promoted innovative drugs, guided pharmaceutical enterprises to intensify innovation and improve competitiveness from the demand side, and promoted the innovative development of the pharmaceutical industry. More and more high-quality innovative drugs have been included in the National Reimbursement Drug List. For patients, including innovative drugs in medical insurance at a reasonable price not only makes medicine available and good drugs accessible, but also reduces the burden of treatment and enables patients to make personalized drug choices.
In the future, Baheal Pharma will actively respond to national policies, focus on R&D and production of high-end drugs, and bring more international quality drugs with superior efficacy and reliable safety to domestic patients, while improving the R&D level and clinical effectiveness of domestic drugs.
-
Baheal Medical Inc. Lands on ChiNext Board Today!
2021-06-30 -
2021-11-09
-
Baheal Medical New Retail Business: Cute Camel Store 2.0 Opened in Qingdao
2021-03-20 -
2021-11-26
-
BAHEAL Pharmaceutical Group Accepted by International Federation of Pharmaceutical Wholesalers
2016-04-05 -
Baheal Receives Four Honors at China Future Healthcare Rankings Top 100
2021-04-17 -
Baheal Medical Launches Commercialization Platform of Efficacious Cosmetics
2021-05-13 -
2019-03-29





